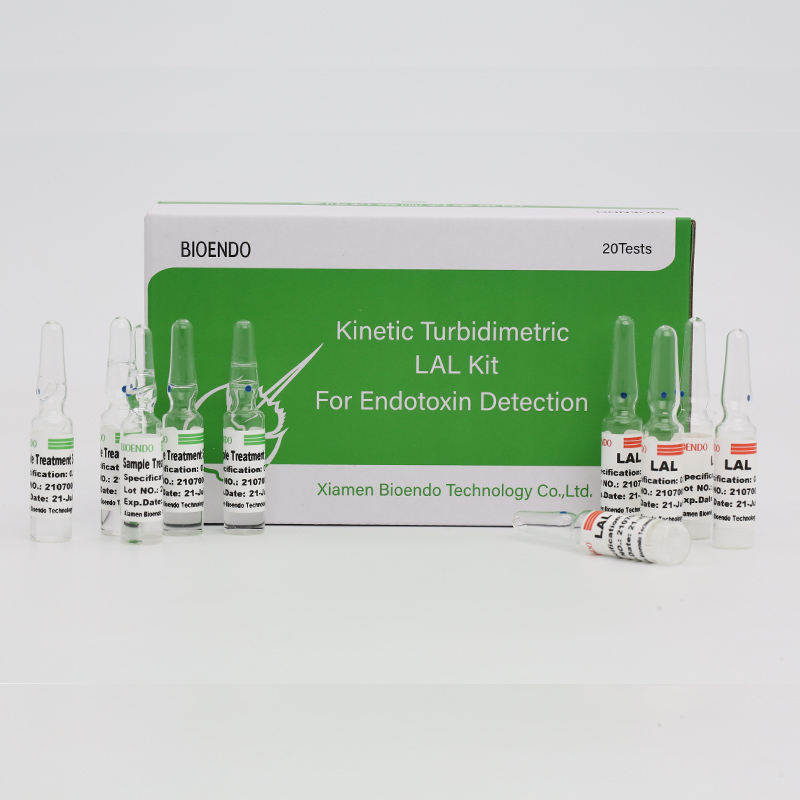
Ngwa Endotoxin Assay maka Plasma mmadụ
Endotoxin Assay Kitmaka mmadụ Plasma
1. Ngwaahịa Ozi
CFDA kpochapụrụNgwa nyocha nke endotoxin Clinicalna-ebelata ọkwa endotoxin na plasma ọbara.Endotoxin bụ isi ihe dị na mgbidi cell nke nje bacteria Gram Negative ma bụrụ onye mgbasa ozi microbial kachasị mkpa nke sepsis.Ọkwa endotoxin dị elu nwere ike ịkpata ahụ ọkụ, mgbanwe na ọnụ ọgụgụ sel ọbara ọcha yana, n'ọnọdụ ụfọdụ, ujo obi.Ọ dabere na ihe kpatara Cpathway na limulus Polyphemus (ọbara nshịkọ inyinya).Na kinetic microplate reader na endotoxin assay software, Endotoxin assay kit na-achọpụta ọkwa endotoxin na plasma mmadụ n'ihe na-erughị otu awa.Ngwa ahụ na-abịa na reagent tupu ọgwụgwọ plasma nke na-ewepụ ihe mgbochi na plasma n'oge nyocha endotoxin.
2. Product Parameter
Oke nyocha: 0.01-10 EU/ml
3. Njirimara ngwaahịa na ngwa
Na-abịa na ngwọta pretreatment plasma, na-ewepụ ihe mgbochi na plasma mmadụ.
Mara:
Lyophilized Amebocyte Lysate (LAL) reagent nke Bioendo rụpụtara bụ nke sitere na amebocyte lysate enwetara ọbara nke nshịkọ akpụkpọ ụkwụ.
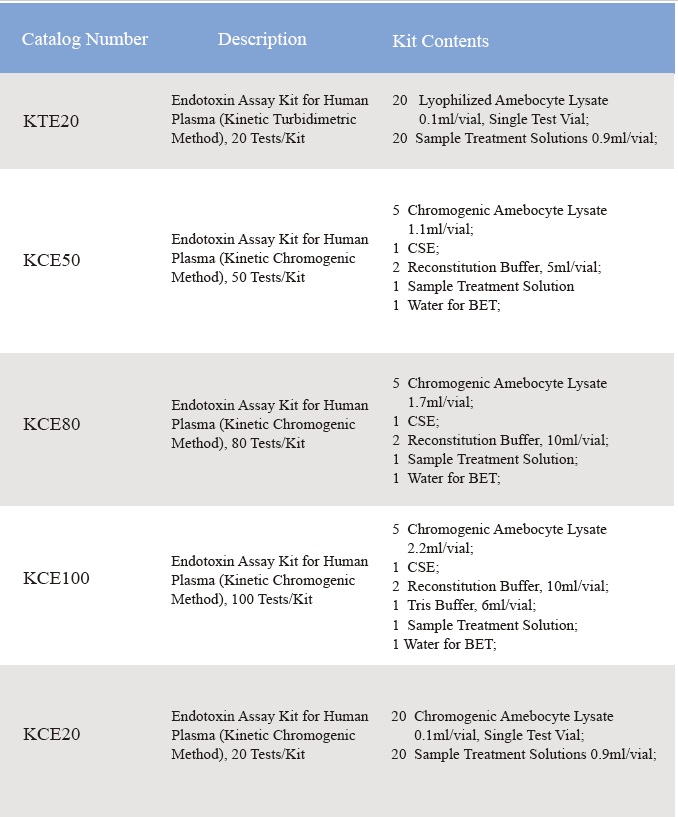
A na-enyocha uche nke Lyophilized Amebocyte Lysate na ike nke Control Standard Endotoxin megide USP Reference Standard Endotoxin.Ngwa Lyophilized Amebocyte Lysate reagent ngwa na-abịa na ntuziaka ngwaahịa, Asambodo nyocha, MSDS.